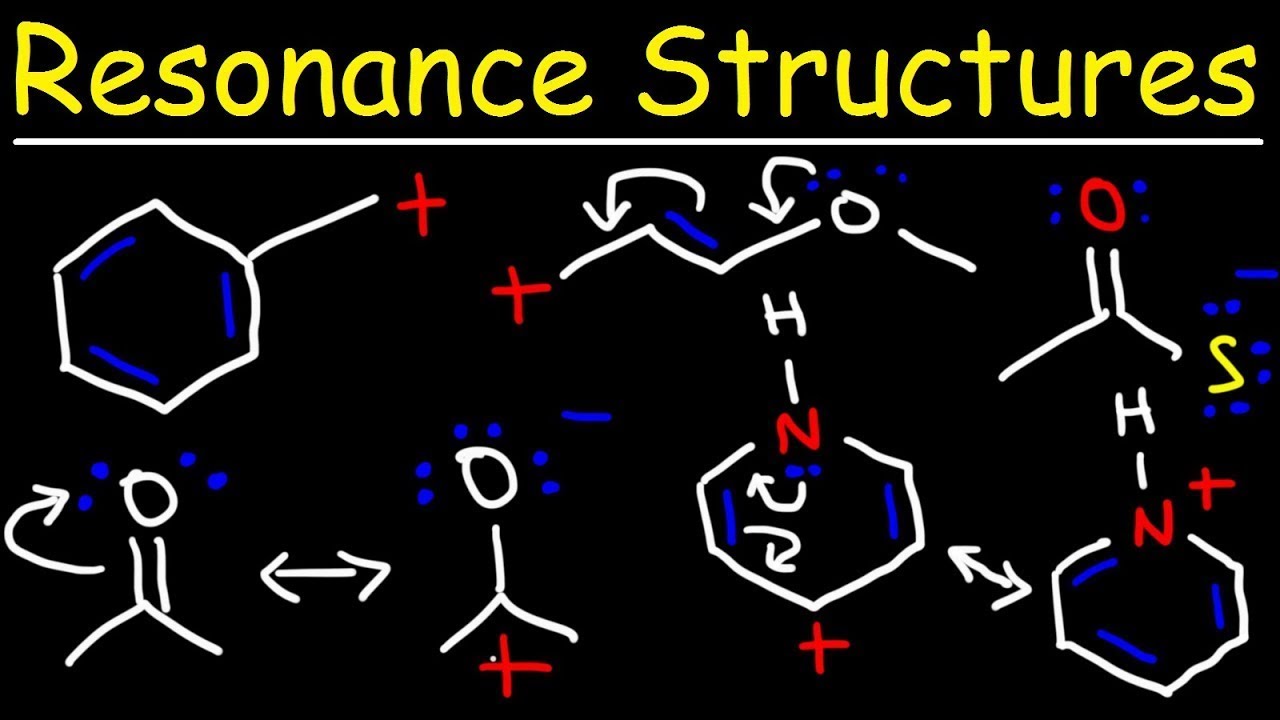
Introduction to Resonance
Definition of Resonance
Resonance is a concept used in chemistry to describe the delocalization of electrons within certain molecules or polyatomic ions. It is a way to represent the bonding that cannot be fully expressed by a single Lewis formula. In resonance, multiple Lewis structures are constructed, each of which represents a possible arrangement of electrons within the molecule. These structures are called resonance structures.
Importance of Resonance in Chemistry
Resonance is an important concept in chemistry as it helps in understanding the electronic structure and bonding of molecules. Here are some key points highlighting the significance of resonance:
1. **Describing Delocalization of Electrons**: Resonance allows us to describe the delocalization of electrons within a molecule or ion. It helps in understanding how the electrons are distributed and shared among different atoms.
2. **Multiple Resonance Structures**: In cases where a single Lewis structure cannot fully explain the bonding, resonance provides a way to represent the alternative arrangements of electrons. Each resonance structure contributes to the overall description of the molecule, and the actual electronic structure is a combination of all the resonance structures, called a resonance hybrid.
3. **Stabilization of Molecules**: Resonance can lead to the stabilization of molecules or ions. This stabilization occurs when electron delocalization lowers the overall energy of the system. For example, in the benzene molecule, the delocalization of π electrons across the ring contributes to its stability.
4. **Reactivity and Bonding**: The presence of resonance can affect the reactivity and bonding properties of molecules. Resonance structures can influence the polarity of bonds, determine the distribution of charges, and impact the stability of intermediates in chemical reactions.
5. **Explanation of Bond Lengths**: Resonance can help explain the phenomenon of bond lengths that are intermediate between single and double bonds. For example, in the nitrate ion (NO3-), the bond lengths between the nitrogen and oxygen atoms are equal due to resonance, even though double and single bonds are involved.
In conclusion, resonance is a powerful concept in chemistry that describes the delocalization of electrons within molecules or ions. It allows for a more complete understanding of the electronic structure and bonding in complex systems. Resonance structures provide a way to represent multiple possible arrangements of electrons, which contribute to the overall description of the molecule. The concept of resonance is essential for explaining the stability, reactivity, and bonding properties of various compounds.
Lewis Structures and Resonance
Lewis Structures and their Limitations
Lewis structures are a way to represent the arrangement of atoms and electrons in a molecule. They are based on the concept of valence electrons, which are the outermost electrons involved in bonding. In a Lewis structure, dots or lines are used to represent electrons, while atoms are represented by their chemical symbols.
However, Lewis structures have some limitations. They are based on the assumption that all atoms in a molecule have a fixed position and that the electrons are localized between the atoms. This means that Lewis structures cannot fully describe the bonding in molecules where the electrons are not localized.
Introduction to Resonance Structures
Resonance structures are used when a single Lewis structure cannot fully describe the bonding in a molecule. They represent different ways of arranging the electrons within the molecule while maintaining the same overall connectivity of atoms.
The concept of resonance can be understood as a mental exercise to describe the delocalization of electrons within a molecule. It involves constructing multiple Lewis structures, called resonance contributors, that when combined represent the full electronic structure of the molecule. The combination of these resonance contributors is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule.
Resonance structures are especially important in molecules with double bonds or lone pairs of electrons. For example, in the case of the nitrite ion (NO2-), a single Lewis structure would suggest that one nitrogen-oxygen bond is shorter and stronger than the other. However, when resonance is considered, we can see that the double bond can be delocalized between both oxygen atoms, resulting in an average bond length and strength between the two.
By considering resonance structures, we can gain a better understanding of the electronic structure and bonding in molecules. This understanding is crucial in predicting the reactivity and properties of different compounds.
In conclusion, Lewis structures have limitations when it comes to describing the bonding in molecules with delocalized electrons. Resonance structures provide a more accurate representation by considering different arrangements of electrons within the molecule. By understanding resonance, chemists can better predict and explain the behavior of molecules in various chemical reactions.
The Concept of Delocalized Electrons
Understanding Delocalized Electrons
Delocalization refers to the spread of electrons over a larger area instead of being confined to a specific region in a molecule. In chemical bonding, delocalized electrons have a significant impact on the stability and reactivity of molecules.
In a molecule, valence electrons can be either localized or delocalized. Localized electrons are confined to a specific bond between two atoms, whereas delocalized electrons are not associated with any particular bond. Instead, they are spread out over multiple atoms or molecular orbitals.
Delocalized electrons are often found in molecules with conjugated systems, which are arrangements of alternating single and multiple bonds. These electrons are able to move freely across the conjugated system, resulting in enhanced stability and reactivity.
Significance of Delocalization in Resonance
Delocalization plays a crucial role in resonance, which is a phenomenon observed in molecules when a single Lewis structure cannot fully describe the bonding. Resonance structures are used to represent the different ways in which electrons can be delocalized within a molecule.
When constructing resonance structures, multiple Lewis structures are created, each representing a different arrangement of electrons. These resonance contributors are combined to form a resonance hybrid, which represents the overall delocalization of electrons in the molecule.
The significance of delocalization in resonance lies in its stabilizing effect. Delocalized electrons are able to spread charge density over a larger area, reducing the energy associated with localized charges. This leads to enhanced stability and lowers the overall energy of the molecule.
In addition to stability, delocalization also affects the reactivity of molecules. Delocalized electrons are more mobile and can participate in chemical reactions more readily. This allows for greater reactivity and versatility in the behavior of molecules.
Understanding the concept of delocalized electrons is essential for predicting and explaining the behavior of molecules in various chemical reactions. It provides insights into the electronic structure, stability, and reactivity of compounds.
In conclusion, delocalization of electrons is a fundamental concept in chemistry that plays a crucial role in resonance and the stability of molecules. This concept highlights the importance of considering multiple arrangements of electrons, rather than relying solely on a single Lewis structure, to accurately describe the bonding in complex molecules. By understanding delocalization, chemists can gain a better understanding of the properties and behavior of different compounds.
Representing Resonance Structures
How to Draw Resonance Structures
When drawing resonance structures, it is important to follow a set of guidelines to accurately represent the delocalization of electrons within a molecule. Here are the steps to draw resonance structures:
1. Identify the atoms involved: Circle the atoms that are directly involved in the resonance phenomenon. These are usually atoms with unshared electron pairs or atoms connected by double or triple bonds.
2. Determine the connectivity: Keep the connectivity of the atoms the same in all resonance structures. This means that the bonds between the atoms should remain unchanged.
3. Move electrons: Use curved arrows to show the movement of electrons between atoms. These arrows indicate the flow of electrons from areas of higher electron density to areas of lower electron density. Remember that electrons should always move towards a positively charged atom or a region where a positive charge is forming.
4. Draw alternate arrangements: Using the information from the curved arrows, draw different arrangements of electrons while keeping the same connectivity of atoms. Each Lewis structure represents a resonance contributor.
5. Include formal charges: Check the formal charges on each atom in each resonance structure. It is important to have the overall charge of the molecule or ion balanced, and to minimize formal charges as much as possible.
By following these steps, you can accurately represent resonance structures and illustrate the delocalization of electrons within a molecule or ion.
Identifying Major and Minor Resonance Contributors
Not all resonance structures are equal in stability. Some resonance contributors, known as major contributors, are more stable than others. Major resonance structures are the ones that contribute more to the overall electronic structure of the molecule.
To determine the major resonance contributors, follow these rules:
1. Complete octets: Resonance structures that have all atoms with a complete octet of electrons are generally more stable.
2. Minimize formal charges: Resonance structures with fewer formal charges, or those with formal charges closer to zero, are more stable.
3. Place charges on more electronegative atoms: Resonance structures that place a negative charge on a more electronegative atom are generally more stable.
By analyzing the resonance structures and applying these rules, you can identify the major and minor contributors. The major contributors will have a greater impact on the overall electronic structure and, therefore, on the physical and chemical properties of the molecule or ion.
In summary, drawing resonance structures involves following specific steps to accurately represent the delocalization of electrons within a molecule. Identifying major and minor resonance contributors helps determine the stability and overall electronic structure of the molecule. By understanding resonance, chemists can gain insights into the reactivity and properties of different compounds.
Resonance in Organic and Inorganic Molecules
Applications of Resonance in Organic Chemistry
Resonance is a fundamental concept in organic chemistry and plays a crucial role in understanding the structure, stability, and reactivity of organic molecules. Here are some key applications of resonance in organic chemistry:
1. Stability of Delocalized Systems: Resonance can stabilize molecules by delocalizing electrons and spreading out their charge density. This stabilization is particularly important in conjugated systems, such as aromatic compounds like benzene. The delocalized pi bonds in benzene give it stability and unique properties.
2. Understanding Bonding and Reactivity: Resonance structures help explain bond lengths and strengths, as well as reactivity patterns of organic molecules. For example, the alternating single and double bonds in the resonance structures of a carboxylate ion contribute to its acidity and ability to form resonance-stabilized intermediates.
3. Electron Delocalization in Radical Species: Resonance can also occur in radical species, where unpaired electrons are delocalized across a molecule. This delocalization stabilizes the radical and affects its reactivity. Examples include resonance-stabilized radicals like the benzyl radical.
4. Predicting Molecular Properties: Resonance structures help in predicting molecular properties such as dipole moments, acidity/basicity, and stability. By considering the various resonance contributors, chemists can determine the most stable resonance hybrid, which reflects the overall electron distribution in the molecule.
Examples of Resonance in Inorganic Molecules
Resonance is not limited to organic molecules; it also plays a role in understanding the bonding and properties of inorganic compounds. Here are some examples:
1. Nitrate Ion (NO3-): The nitrate ion is a classic example of resonance in inorganic chemistry. It can be represented by three resonance structures, where the negative charge is delocalized over the three oxygen atoms. This delocalization contributes to the stability of the nitrate ion.
2. Carbonate Ion (CO32-): The carbonate ion also exhibits resonance and can be represented by two major resonance structures. This resonance stabilization contributes to the stability of carbonates and their involvement in important geological processes, such as the formation of limestone.
3. Sulfate Ion (SO42-): Similarly, the sulfate ion can be represented by resonance structures where the negative charge is delocalized over the four oxygen atoms. This delocalization enhances the stability of sulfate and its role in various chemical processes.
4. Metal-Ligand Bonding: Resonance can also influence metal-ligand bonding in coordination compounds. The delocalization of electrons in ligands can affect the strength and nature of the metal-ligand bond, leading to different properties and reactivity.
In conclusion, resonance is a powerful concept in both organic and inorganic chemistry. It helps explain the stability, reactivity, and properties of molecules by considering the delocalization of electrons. Resonance structures provide valuable insights into the nature of chemical bonding and are essential tools for understanding and predicting the behavior of organic and inorganic compounds.
Resonance Hybridization
Definition and Characteristics of Resonance Hybridization
Resonance hybridization is a concept in chemistry that describes the mixing of different resonance structures to form a hybrid structure. It is an important concept within the Valence Bond Theory of bonding. In resonance hybridization, multiple Lewis structures are combined to represent the full electronic structure of a molecule.
The main characteristic of resonance hybridization is the delocalization of electrons within the molecule. The delocalization occurs because the electrons are not confined to a single location or bond but are spread out over multiple atoms. This leads to the formation of a more stable overall structure.
Resonance hybridization allows us to better understand the bonding in molecules that cannot be fully described by a single Lewis structure. It provides a more accurate representation of the electronic structure and helps explain the properties and reactivity of these molecules.
Predicting Bond Lengths and Energies using Resonance Hybridization
Resonance hybridization also has implications for predicting bond lengths and energies in molecules. The delocalization of electrons leads to a redistribution of electron density, which affects the strength and length of chemical bonds.
When a molecule has resonance structures, the bond lengths are typically intermediate between the lengths predicted by the individual Lewis structures. This is because the delocalization of electrons spreads the electron density over a larger region, resulting in a longer bond length compared to a single bond.
Similarly, the energy associated with the bonds in a resonance hybrid is lower than that of any individual Lewis structure. This is due to the sharing of electron density and the resulting stabilization of the molecule.
Predicting bond lengths and energies using resonance hybridization is important for understanding the physical and chemical properties of molecules. It allows us to make educated predictions about the strength of bonds, the stability of compounds, and their reactivity in chemical reactions.
In conclusion, resonance hybridization is a concept that describes the delocalization of electrons within molecules. It involves combining multiple resonance structures to form a hybrid structure that better represents the overall electronic structure of the molecule. Resonance hybridization provides insights into the stability, bond lengths, and energies of molecules, helping chemists understand their properties and behavior.
Stability and Energy of Resonance Structures
Comparing the Stability of Resonance Structures
Resonance structures are different representations of a molecule or ion that show the distribution of delocalized electrons. When comparing the relative stability of resonance structures, the lower the energy, the more stable the structure is.
One guideline for determining the stability of resonance structures is to consider the octet rule. In general, structures with complete octets are more stable. However, there are exceptions to this rule that are discussed in section 2.4 of Organic Chemistry I (Liu).
Another factor that affects the stability of resonance structures is the distribution of formal charges. Resonance structures with minimized formal charges or with negative charges on more electronegative atoms tend to be more stable.
In some cases, the stability of a resonance structure can be determined by the extent of electron delocalization. Structures with greater electron delocalization are more stable because the delocalization spreads the electron density over a larger region, resulting in a lower overall energy.
Understanding Resonance Energy and its Significance
Resonance energy is the difference in energy between the most stable resonance structure and the contributing resonance structures. It is a measure of the stability of a molecule or ion with delocalized electrons.
The more stable the contributing resonance structures, the higher the resonance energy. Resonance energy is a significant concept in chemistry because it helps explain the enhanced stability of molecules with multiple resonance structures.
Resonance energy is also related to the reactivity of a molecule. Molecules with higher resonance energy are generally less reactive because they are more stable. This stability reduces their tendency to undergo chemical reactions.
Predicting bond lengths and energies using resonance hybridization is important for understanding the physical and chemical properties of molecules. It allows chemists to make educated predictions about the strength of bonds, the stability of compounds, and their reactivity in chemical reactions.
In conclusion, the stability of resonance structures is determined by factors such as octet rule compliance, distribution of formal charges, and extent of electron delocalization. Resonance energy, which measures the stability of a molecule or ion with delocalized electrons, is an important concept that impacts the reactivity of molecules. Understanding and utilizing resonance hybridization helps chemists better understand the properties and behavior of molecules with multiple resonance structures.
Limitations and Exceptions of Resonance
Cases where Resonance is not Applicable
– Resonance is a powerful concept in understanding the electronic structure of molecules, but it has its limitations. There are certain cases where resonance is not applicable or does not accurately describe the bonding in a molecule.
– In molecules with highly reactive species, such as free radicals or compounds with odd numbers of electrons, resonance structures may not fully capture the true electronic structure.
– Resonance is based on the assumption of static structures, where the bonding remains fixed. However, in some cases, molecules may undergo dynamic processes, such as bond breaking and formation, that cannot be fully described by resonance structures.
– Additionally, resonance assumes that all resonance structures contribute equally to the overall structure. In reality, certain structures may contribute more significantly than others, leading to a deviation from the resonance hybrid.
– Lastly, resonance is not applicable to non-bonding interactions or in cases where electron-electron repulsion dominates over electron delocalization.
Complex Examples that Challenge Resonance Concepts
– There are also complex examples that challenge the concepts of resonance. These are cases where molecules exhibit unique bonding that cannot be fully explained using traditional resonance theory.
– One example is the concept of hyperconjugation, which involves the delocalization of electron density from a σ or π bond into an adjacent empty or partially filled orbital. This concept explains the stability and reactivity of certain compounds, such as carbocations, beyond what can be accurately described by resonance structures.
– Another example is the π-acceptor/donor interactions in transition metal complexes. These interactions involve the donation or acceptance of electron density from or to a metal center, resulting in unique bonding and reactivity that cannot be fully explained by simple resonance structures.
– Additionally, there are cases where aromaticity and anti-aromaticity play a significant role in the bonding of certain compounds. These concepts go beyond the standard rules of resonance and require a deeper understanding of molecular orbital theory.
– These complex examples highlight the need for a more comprehensive approach to understanding bonding in molecules, taking into account additional factors such as orbital overlap, electronic effects, and the overall molecular symmetry.
In conclusion, while resonance is a valuable tool for understanding the electronic structure of molecules, it has limitations and exceptions. There are cases where resonance is not applicable or does not fully describe the bonding in a molecule. Complex examples challenge the concepts of resonance and require a more comprehensive understanding of molecular bonding. Continued research and advancements in theoretical chemistry are necessary to further our understanding of these complex phenomena.
Conclusion
Recap of Resonance Concepts
– Resonance is a mental exercise within the Valence Bond Theory that describes the delocalization of electrons within molecules.
– Multiple Lewis structures are constructed to represent the full electronic structure of a molecule.
– Resonance structures are used when a single Lewis structure cannot fully describe the bonding, and the combination of these structures forms a resonance hybrid.
– Resonance allows for the understanding of complex bonding situations and the overall delocalization of electrons within a molecule.
Importance of Understanding Resonance in Chemical Bonding
– Resonance is a powerful tool in understanding the electronic structure of molecules and predicting their chemical behavior.
– It provides insight into the stability and reactivity of compounds, allowing chemists to design efficient synthetic routes and optimize reaction conditions.
– Understanding resonance helps explain the properties of aromatic compounds, which are essential in organic chemistry.
– Resonance is also crucial in the study of reaction mechanisms, as it helps identify key intermediates and transition states.
– It allows chemists to predict the relative strength of bonds and the distribution of electron density within a molecule.
– Mastering the concepts of resonance provides a solid foundation for further exploration of advanced topics in theoretical and computational chemistry.
In conclusion, understanding resonance is of utmost importance in the field of chemical bonding. It allows chemists to decipher the complex electronic structures and predict the behavior of molecules. Despite its limitations and exceptions, resonance provides valuable insights into the nature of bonding and the distribution of electrons. Developing a strong grasp of resonance concepts lays the groundwork for a deeper understanding of molecular properties and reactivity. Continued research and advancements in theoretical chemistry will further enhance our understanding of resonance and its applications in various fields of chemistry.