Introduction
Overview of causes and progenitors in biological processes
Understanding the role of embryonic progenitors in relation to striatal development is crucial for advancing our understanding of striatal dysfunction in neurodevelopmental and neurodegenerative disorders. Recent evidence suggests that defects in the division and differentiation of these progenitors are associated with diseases such as Huntington’s disease and autism spectrum disorder. Stem cells play a significant role in this context.
Progenitor cells, which are lineage-restricted and have limited proliferative capacity, exhibit two essential properties. Firstly, they have the ability to self-renew, meaning they can divide to produce more progenitor cells. Secondly, they have the ability to differentiate into different types of cells. Stem cells are often categorized based on their potency: totipotent, pluripotent, multipotent, or unipotent.
Impact of understanding causes and progenitors in various fields of study
1. **Neurodevelopmental Disorders**: By studying the causes and behavior of embryonic progenitors, we can gain insights into the underlying mechanisms of neurodevelopmental disorders such as autism spectrum disorder. Understanding defects in the division and differentiation of progenitors could potentially lead to the development of targeted therapies for these disorders.
2. **Neurodegenerative Disorders**: Huntington’s disease is one example of a neurodegenerative disorder associated with defects in embryonic progenitors. Investigating the role of these progenitors in the development of the striatum might provide new avenues for therapeutic interventions to alleviate the symptoms and progression of such diseases.
3. **Regenerative Medicine**: Progenitor cells have the potential to be used in regenerative medicine. By understanding their behavior and ability to differentiate into different cell types, researchers can develop strategies to generate functional mature neural cells for transplantation. This could have significant implications for the treatment of neurological disorders and injuries.
4. **Drug Development**: Studying progenitor cells and their role in striatal development can help researchers identify potential targets for drug development. By understanding the molecular pathways involved in the division and differentiation of progenitors, scientists can develop drugs that specifically target these processes, potentially leading to more effective treatments for neurological disorders.
In conclusion, understanding the causes and behavior of embryonic progenitors is essential for advancing our knowledge of various biological processes and their implications in different fields of study. The ability to manipulate progenitor cell development could help generate functional mature neural cells and provide insights into the pathophysiology of several disorders. This knowledge has the potential to contribute to the development of targeted therapies and improve the overall understanding and treatment of neurological disorders.
Genetic Causes
Explanation of genetic causes and their role in different diseases or conditions
Genetic disorders are caused by mutations or changes in the genes. These mutations can either be inherited from the parents or occur spontaneously. The genetic material responsible for transmitting traits from one generation to another is called DNA. Within the DNA, specific sequences called genes provide instructions for making proteins, which are essential for various cell functions. When a mutation occurs in a gene, it can alter the protein-making instructions and disrupt normal cellular processes. This disruption can lead to the development of genetic disorders.
There are different types of genetic causes for disorders. Some genetic disorders are caused by mutations in a single gene, known as monogenic disorders. Examples of monogenic disorders include cystic fibrosis and sickle cell anemia. These disorders are typically inherited in an autosomal recessive or autosomal dominant manner. In autosomal recessive disorders, both copies of the gene must have mutations for the disorder to be present, while in autosomal dominant disorders, a single copy of the mutated gene is sufficient for the disorder to manifest.
Other genetic disorders are caused by changes in the structure or number of chromosomes, known as chromosomal disorders. These alterations can involve missing or duplicated chromosome material and can result in conditions such as Down syndrome and Turner syndrome. Chromosomal disorders typically occur spontaneously and are not inherited.
Examples of genetic causes and their implications
One example of a genetic cause is mutations in the BRCA1 and BRCA2 genes, which are associated with an increased risk of developing breast and ovarian cancers. Individuals with certain mutations in these genes have a higher likelihood of developing these types of cancer compared to the general population. Genetic testing can help identify individuals who have these mutations, allowing for early detection and proactive measures to reduce the risk.
Another example is the mutation in the CFTR gene, which causes cystic fibrosis. This genetic disorder affects the lungs, digestive system, and other organs. The abnormal CFTR protein, resulting from the mutation, leads to the production of thick and sticky mucus, causing breathing difficulties and other complications. Understanding the genetic cause of cystic fibrosis has paved the way for targeted treatments and therapies specific to the underlying mutation.
By identifying the genetic causes of various disorders, researchers and healthcare professionals can gain insights into disease mechanisms, develop targeted therapies, and provide genetic counseling to individuals and families affected by these conditions. Genetic testing plays a crucial role in diagnosing genetic disorders and guiding medical interventions, enabling personalized medicine approaches that can improve patient outcomes.
Environmental Causes
Discussion on environmental factors that contribute to the development of diseases or conditions
Environmental factors play a significant role in the development of various diseases and conditions, including cancer. These factors can encompass a wide range of exposures, such as chemicals, pollutants, radiation, lifestyle choices, and occupational hazards. Understanding the impact of these environmental causes is crucial for preventing and managing diseases effectively.
Studies have shown that exposure to certain environmental factors can increase the risk of developing cancer. For example, prolonged exposure to tobacco smoke, both active and passive, is a significant risk factor for lung cancer. Similarly, exposure to asbestos fibers can lead to mesothelioma, a rare type of cancer that affects the lining of the lungs and other organs.
Other environmental factors, such as exposure to ultraviolet (UV) radiation from the sun or tanning beds, contribute to the development of skin cancer. Chronic exposure to certain chemicals found in pesticides, industrial pollutants, and household products may also increase the risk of developing various types of cancer, including breast, prostate, and bladder cancer.
In addition to cancer, environmental factors can also contribute to the development of other health conditions. For instance, air pollution has been linked to respiratory diseases, cardiovascular problems, and even neurological disorders. Poor diet, high in processed foods and low in fruits and vegetables, along with sedentary lifestyles, can lead to obesity, diabetes, and cardiovascular diseases.
Specific examples of environmental causes and their effects on health
1. Air Pollution: Exposure to high levels of air pollution, such as fine particulate matter, can lead to respiratory issues, including asthma, bronchitis, and chronic obstructive pulmonary disease (COPD). Long-term exposure has also been associated with an increased risk of heart disease, stroke, and lung cancer.
2. Pesticide Exposure: Agricultural workers and those living in close proximity to farms are at a higher risk of exposure to pesticides. Studies have shown that certain pesticides, such as organophosphates and glyphosate, may be linked to an increased risk of cancer, neurodevelopmental disorders, and hormonal imbalances.
3. Occupational Hazards: Workers in certain industries, such as construction, mining, and manufacturing, may be exposed to hazardous substances and carcinogens. For example, asbestos exposure in construction and insulation work can lead to mesothelioma and other lung diseases. Similarly, exposure to benzene, a chemical found in gasoline and industrial solvents, is associated with an increased risk of leukemia and other blood-related cancers.
4. Radon Exposure: Radon is a radioactive gas that can be found in homes, particularly in areas with high levels of uranium in the soil. Prolonged exposure to radon gas increases the risk of lung cancer.
5. UV Radiation: Overexposure to UV radiation from the sun or tanning beds can damage the DNA in skin cells, leading to skin cancer, including melanoma, the most severe form of skin cancer.
By understanding the specific environmental causes and their effects on health, individuals and communities can take proactive measures to minimize exposures and reduce the risk of developing diseases or conditions. This can include adopting a healthy lifestyle, avoiding known carcinogens, implementing proper safety measures in the workplace, and advocating for stricter regulations to protect public health.
Epigenetic Causes
Explanation of epigenetic causes and their influence on gene expression
Epigenetics refers to the study of heritable changes in gene activity (expression) that do not involve alterations to the DNA sequence itself. These changes can be caused by various factors, including environmental influences such as nutrition, stress, and exposure to toxins. Epigenetic modifications can alter the structure of DNA or the proteins associated with DNA, affecting gene expression and ultimately impacting cellular function.
Epigenetic modifications can occur through different mechanisms, such as DNA methylation and histone modification. DNA methylation involves the addition of a chemical group called a methyl group to specific regions of DNA, which can result in the silencing of gene expression. Histone modification, on the other hand, refers to changes in the proteins called histones that help package DNA within the nucleus. These modifications can either activate or repress gene expression, depending on the specific modification.
The role of epigenetics in understanding disease development
Epigenetic modifications play a crucial role in normal development and cellular differentiation. However, abnormalities in these modifications can contribute to the development of various diseases, including cancer, cardiovascular diseases, and neurological disorders. Epigenetic alterations can affect gene expression patterns, leading to dysregulated cellular processes and disease phenotypes.
For example, aberrant DNA methylation patterns have been observed in many types of cancer. Hypermethylation of tumor suppressor genes can lead to their silencing, allowing uncontrolled cell growth and the formation of tumors. Histone modifications, such as acetylation and methylation, have also been implicated in cancer development, as they can affect gene expression associated with cell proliferation, apoptosis, and DNA repair.
In addition to cancer, epigenetics plays a role in cardiovascular diseases. Studies have shown that DNA methylation patterns can be altered in response to environmental factors, such as smoking or high-fat diets, leading to changes in gene expression related to inflammation and vascular health. These epigenetic changes can contribute to the development of conditions such as atherosclerosis and hypertension.
Furthermore, epigenetic modifications have also been implicated in neurological disorders, such as Alzheimer’s disease and autism spectrum disorders. Abnormal DNA methylation patterns and histone modifications have been observed in the brains of individuals with these conditions, affecting the expression of genes involved in synaptic function, neuronal development, and neurotransmitter signaling.
Understanding the role of epigenetics in disease development is crucial for the development of targeted therapies and preventive strategies. Researchers are exploring the potential of epigenetic modifiers, such as DNA methylation inhibitors and histone deacetylase inhibitors, as therapeutic agents that can reverse abnormal epigenetic changes and restore normal gene expression. These epigenetic therapies hold promise for the treatment of various diseases and may provide new avenues for personalized and precision medicine approaches.
In conclusion, epigenetics bridges the gap between genetic and environmental factors in the development of diseases. Genetic causes contribute to the susceptibility of individuals to certain disorders, while epigenetic alterations can modulate gene expression and disease phenotypes. Understanding both genetic and epigenetic factors is essential for unraveling the complexity of diseases and developing targeted treatments that account for the interplay between genes and the environment.
Developmental Causes
Overview of developmental causes and their impact on health and well-being
Developmental disabilities can occur due to a wide range of causes, including genetic factors and environmental exposures. These causes can have a significant impact on an individual’s health and well-being, affecting their physical, cognitive, and emotional development.
Genetic factors play a crucial role in the development of many developmental disabilities. Certain genetic mutations or variations can disrupt normal development and result in conditions such as Down syndrome, Fragile X syndrome, and autism spectrum disorders. These genetic causes are often present from birth and can have lifelong implications for individuals and their families.
In addition to genetic causes, environmental exposures can also contribute to the development of developmental disabilities. Factors such as maternal smoking and drinking during pregnancy, infections during pregnancy, and exposure to environmental toxins like lead can increase the risk of developmental disabilities. These exposures can interfere with normal fetal development and result in a range of impairments, including intellectual disabilities, learning disorders, and behavioral challenges.
Examples of developmental causes and their consequences
Low birthweight, premature birth, multiple births, and infections during pregnancy are all examples of developmental causes that can have significant consequences. These factors can increase the risk of various developmental disabilities and have long-term effects on a child’s health and development.
Low birthweight, defined as weighing less than 5.5 pounds at birth, can result from various factors, including preterm birth and growth restrictions. Low birthweight infants are at a higher risk of developmental delays, cognitive impairments, and physical disabilities. They may require special interventions and therapies to support their development and improve their long-term outcomes.
Premature birth, defined as birth before 37 weeks gestation, can also lead to developmental disabilities. Premature infants often face challenges related to organ immaturity and may experience difficulties with breathing, feeding, and regulating their body temperature. These factors can impact their overall development and increase the risk of long-term complications, including cognitive impairments and learning disabilities.
Multiple births, such as twins or triplets, are associated with an increased risk of developmental disabilities. Multiple pregnancies can place additional strain on the mother’s body, increasing the risk of complications and preterm birth. The challenges of multiple pregnancies and the increased likelihood of premature birth can contribute to a higher prevalence of developmental delays and disabilities in these children.
Infections during pregnancy, such as rubella, cytomegalovirus (CMV), or Zika virus, can have devastating effects on fetal development. These infections can result in a range of disabilities, including intellectual disabilities, hearing loss, vision problems, and physical impairments. Preventative measures, such as vaccination and practicing good hygiene, are important in reducing the risk of these infections during pregnancy.
Overall, understanding the various developmental causes and their consequences is crucial for early detection, intervention, and support for individuals with developmental disabilities. Early identification and appropriate interventions can help minimize the impact of these causes and support optimal development and well-being for affected individuals. Collaborative efforts between healthcare professionals, researchers, educators, and families are essential in providing comprehensive care and opportunities for individuals with developmental disabilities to reach their full potential.
Stem Cell Progenitors
Explanation of stem cells and their role as progenitors in various tissues and organs
Stem cells are characterized by their unique ability to self-renew and differentiate into different types of cells. They can be categorized into four main types based on their potential to differentiate: totipotent, pluripotent, multipotent, and unipotent.
Totipotent stem cells have the highest level of differentiation potential, as they can give rise to all cell types in the body, including the three germ layers (endoderm, ectoderm, and mesoderm), germ cells (sperm and oocytes), and placental cells. However, totipotent stem cells are only present during the earliest stages of development, such as in the fertilized egg or the cells of the early embryo.
Pluripotent stem cells, on the other hand, can differentiate into all cell types in the body except for placental cells. These cells are found in embryos during the blastocyst stage and are commonly referred to as embryonic stem cells. Pluripotent stem cells have significant potential for regenerative medicine and tissue engineering, as they can be directed to differentiate into specific cell types for transplantation or replacement therapies.
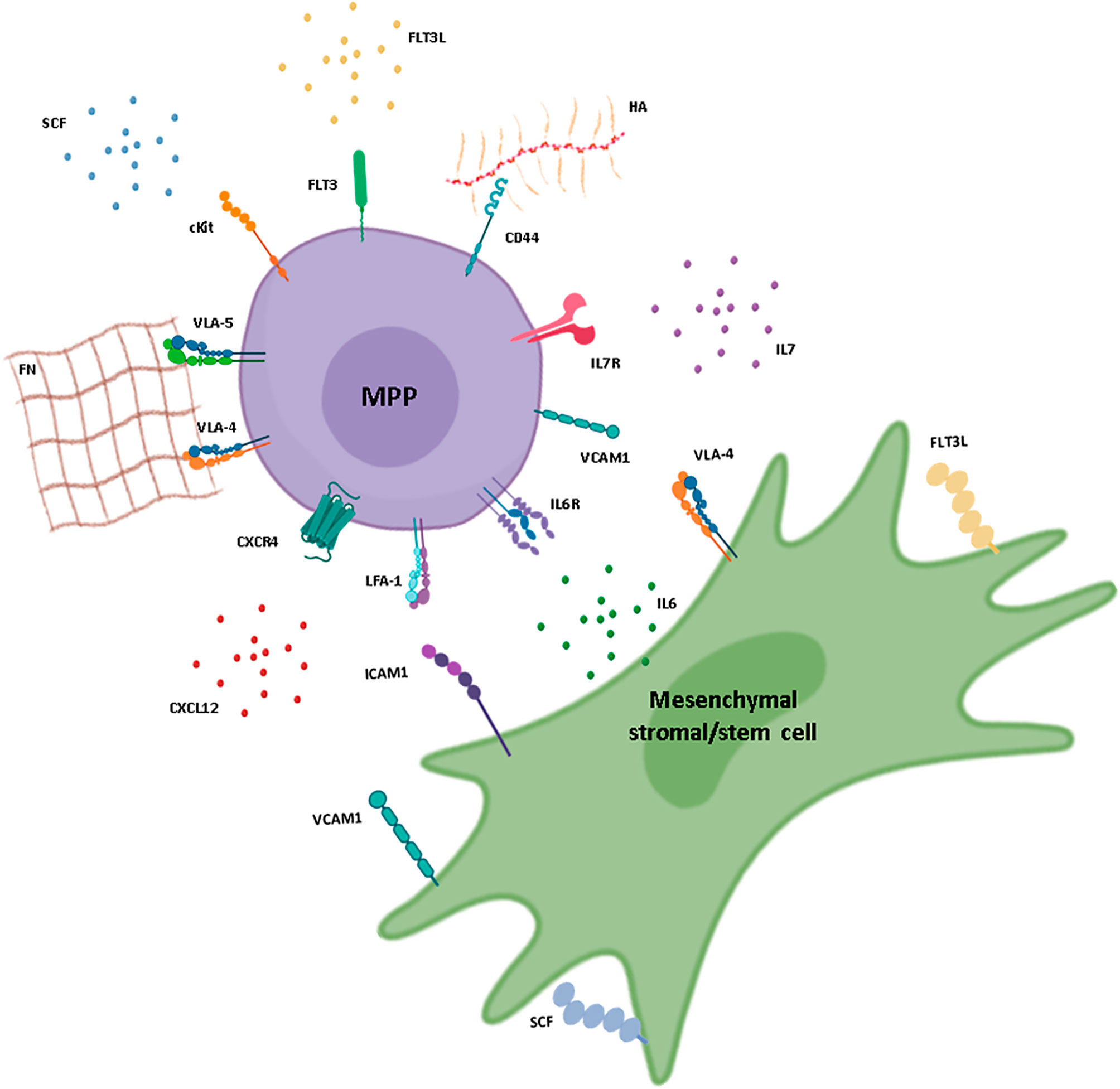
Multipotent stem cells are lineage-restricted based on the organ of origin. These cells have the ability to differentiate into multiple cell types within a specific tissue or organ. For example, hematopoietic stem cells are multipotent and can give rise to various blood cell types, such as red blood cells, white blood cells, and platelets. Similarly, mesenchymal stem cells found in the bone marrow can differentiate into bone cells, cartilage cells, and fat cells.
Unipotent stem cells have a more limited differentiation potential and can only give rise to a single type of cell within a specific tissue or organ. For instance, spermatogonia in the testes are considered unipotent stem cells, as they can only differentiate into sperm cells.
Stem cells are not only present during embryonic development but also throughout the life cycle in fetuses and adults. In adults, stem cells serve as a reservoir for tissue regeneration and repair. They can be found in various tissues and organs, such as the bone marrow, brain, liver, and skin. These adult stem cells contribute to normal tissue homeostasis and have the potential to repair damaged or injured tissues.
Discussion on the importance of stem cell research in understanding diseases
Stem cell research has gained significant attention due to its potential for understanding diseases and developing regenerative therapies. By studying stem cells, scientists can gain insights into the mechanisms that regulate cell differentiation and tissue development. This knowledge is crucial for understanding the causes of diseases and finding new therapeutic approaches.
Stem cells play a fundamental role in the development and progression of various diseases. For example, abnormal stem cell self-renewal or differentiation can result in the formation of tumors and cancer. By studying cancer stem cells, researchers aim to identify specific markers and signaling pathways that can be targeted for more effective cancer treatments.
Moreover, stem cells can also be used as disease models to study the mechanisms underlying genetic disorders, neurodegenerative diseases, and cardiovascular diseases. By generating patient-specific stem cells from induced pluripotent stem cells (iPSCs), researchers can replicate the disease phenotype in a laboratory setting. This enables the investigation of disease progression, drug screening, and the development of personalized medicine approaches.
In addition to disease modeling, stem cell research is essential for developing regenerative medicine therapies. Stem cells hold great promise for tissue transplantation and organ regeneration, as they have the potential to replace damaged or dysfunctional cells. For instance, hematopoietic stem cell transplantation has been successfully used to treat various blood disorders and cancers.
However, challenges and ethical considerations surround stem cell research, particularly in the case of embryonic stem cells. This has led to the exploration of alternative sources, such as iPSCs and adult stem cells. iPSCs have the advantage of being patient-specific, avoiding the issues of immune rejection and ethical concerns.
In conclusion, stem cells, with their ability to self-renew and differentiate, provide valuable insights into developmental processes, disease mechanisms, and potential therapeutic strategies. Understanding the role of stem cells as progenitors in various tissues and organs is crucial for advancing medical research and developing innovative treatments for a wide range of diseases. Stem cell research holds immense potential for regenerative medicine and personalized therapies, paving the way for a future where debilitating diseases can be effectively treated or even cured.
Erythro-Myeloid Progenitors
Explanation of erythro-myeloid progenitors and their involvement in neurodegenerative diseases
Erythro-myeloid progenitors are a specific type of stem cell that give rise to both erythroid (red blood cells) and myeloid (white blood cells) lineages. These progenitors play a crucial role in hematopoiesis, the process of generating all blood cell types in the body.
Recent studies have also highlighted the role of erythro-myeloid progenitors in neurodegenerative diseases. It has been found that a somatic mutation in these progenitors can lead to the development of neurodegenerative diseases. This discovery opens up new avenues for understanding the link between the hematopoietic system and the central nervous system and may have implications for the development of therapeutic strategies for these diseases.
Studies and findings related to erythro-myeloid progenitors
Several studies have investigated the development and function of erythro-myeloid progenitors. One study published in Development in 1999 examined the development of these progenitors in the yolk sac and embryo proper of mice. The study provided insights into the specific populations of endothelial cells that give rise to erythroid and myeloid progenitors.
Another study published in Cell Stem Cell in 2011 further advanced our understanding of erythro-myeloid progenitors. The study identified distinct populations of endothelial cells as the origin of these progenitors and demonstrated their role in hematopoiesis. This finding contributes to our knowledge of the cellular hierarchy involved in blood cell development.
In addition, a more recent study published in 2017 explored the impact of a somatic mutation in erythro-myeloid progenitors on neurodegenerative disease development. The study identified a specific mutation in these progenitors that resulted in the manifestation of a neurodegenerative disease. This finding suggests a potential link between hematopoiesis and the pathogenesis of certain neurological disorders.
These studies collectively contribute to our understanding of the role of erythro-myeloid progenitors in hematopoiesis and their potential involvement in neurodegenerative diseases. Further research in this field may uncover additional insights into the molecular mechanisms underlying the development of these progenitors, as well as their potential therapeutic applications.
It is important to note that the inclusion of these studies in the National Library of Medicine (NLM) database does not imply endorsement or agreement with their contents by the NLM or the National Institutes of Health. However, their publication in reputable scientific journals indicates the significance of their findings in the scientific community.
In conclusion, erythro-myeloid progenitors are a distinct type of stem cell involved in the development of both red and white blood cells. Recent research has shed light on their role in neurodegenerative diseases and has the potential to pave the way for future advancements in understanding and treating these conditions. Continued studies in this area are crucial for unraveling the complexities of hematopoiesis and its potential implications in various disease processes.
Other Progenitor Cells
Overview of other types of progenitor cells in different tissues and organs
In addition to stem cells, there are various types of progenitor cells found in different tissues and organs of the human body. These progenitor cells are responsible for the ongoing growth and maintenance of specific tissues and organs throughout life.
For example, neural progenitor cells are found in the brain and spinal cord. These cells have the ability to differentiate into various types of neurons and glial cells, which play crucial roles in transmitting signals and supporting the functions of the nervous system.
Hematopoietic progenitor cells are another type of progenitor cells that are found in the bone marrow. These cells give rise to different types of blood cells, including red blood cells, white blood cells, and platelets. Hematopoietic progenitor cells are essential for maintaining a functional immune system and ensuring proper oxygen transportation.
There are also progenitor cells specific to certain organs, such as the liver and skin. Liver progenitor cells have the ability to differentiate into hepatocytes, which are the main functional cells of the liver responsible for detoxification and synthesis of essential molecules. Skin progenitor cells are responsible for the renewal and repair of the skin, ensuring its integrity and barrier function.
Different tissues and organs have their own specific types of progenitor cells, each with unique differentiation potentials and functions. These progenitor cells play a crucial role in the continuous regeneration and maintenance of tissues and organs throughout life.
Importance of studying other progenitor cells in disease research
Studying other progenitor cells in different tissues and organs is important for understanding the mechanisms underlying various diseases and developing effective therapeutic strategies.
For instance, abnormalities in neural progenitor cells have been implicated in neurodevelopmental disorders and neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease. By studying these cells, researchers can gain insights into the underlying causes of these diseases and potentially develop treatments to restore neural function.
Similarly, dysregulation of hematopoietic progenitor cells can lead to various blood disorders and cancers. Understanding the molecular mechanisms that control the differentiation and proliferation of these cells can aid in the development of targeted therapies for diseases such as leukemia and anemia.
Progenitor cells in other organs, such as the liver and skin, also play crucial roles in disease processes. Dysfunction of liver progenitor cells can contribute to liver fibrosis and cirrhosis, while impaired skin progenitor cell function can lead to chronic wounds and skin disorders.
In conclusion, studying other progenitor cells in various tissues and organs is essential for understanding the pathogenesis of different diseases and developing innovative therapeutic approaches. These cells contribute to the normal growth, development, and repair of tissues and organs, and their dysregulation can have significant implications for human health. By unraveling the mechanisms that govern the differentiation and function of progenitor cells, researchers can pave the way for targeted interventions and personalized treatments for a wide range of diseases.
Conclusion
Summary of the importance of understanding causes and progenitors in various biological processes
Understanding the causes and functions of different progenitor cells in various tissues and organs is crucial for advancing our knowledge of biological processes and developing effective treatments for diseases. These progenitor cells, which are responsible for the growth, maintenance, and repair of tissues, have unique differentiation potentials and play vital roles in the body.
Studying neural progenitor cells has provided insights into neurodevelopmental disorders and neurodegenerative diseases. Abnormalities in these cells have been linked to conditions like Alzheimer’s and Parkinson’s disease, highlighting the importance of understanding their behavior and function.
Hematopoietic progenitor cells are essential for maintaining a functional immune system and proper oxygen transportation. Dysregulation of these cells can lead to blood disorders and cancers, making it important to study their regulation and behavior for the development of targeted therapies.
Progenitor cells specific to certain organs, such as liver and skin, also contribute to disease processes. Understanding the mechanisms controlling the differentiation and proliferation of liver progenitor cells can help in the treatment of liver fibrosis and cirrhosis. Similarly, investigating skin progenitor cells can aid in the management of chronic wounds and skin disorders.
Future directions and areas of research in causes and progenitors
Continued research on progenitor cells and their involvement in disease processes holds great promise for the development of innovative therapeutic strategies. Here are some future directions and areas of research in this field:
1. Investigation of molecular mechanisms: Further understanding of the molecular mechanisms that control the differentiation and behavior of progenitor cells is crucial. This knowledge will shed light on various diseases and provide opportunities for targeted interventions.
2. Identification of novel therapeutic targets: Through detailed characterization of progenitor cells, researchers can identify new therapeutic targets for the treatment of diseases. This could involve developing drugs or interventions that specifically target the abnormal behavior or function of progenitor cells.
3. Development of regenerative medicine approaches: Progenitor cells have regenerative potential, and harnessing this ability can open up new possibilities for regenerative medicine. Research may focus on finding ways to manipulate progenitor cells to promote tissue regeneration and repair.
4. Understanding species-specific differences: Investigating species-specific differences in progenitor cells, particularly in the neocortex, can provide insights into the evolutionary expansion of the human brain. This knowledge can help us understand the unique features and functions of the human brain compared to other species.
In conclusion, understanding the causes and functions of progenitor cells in various tissues and organs is essential for advancing our knowledge of biological processes and developing treatments for diseases. Continued research in this field will contribute to the development of innovative therapies and regenerative medicine approaches, improving human health and well-being.